科学技術創成研究院 ゼロカーボンエネルギー研究所
物質理工学院応用化学系原子核工学コース・応用化学コース
鷹尾研究室 (Takao Laboratory)
~核のゴミ、化学のチカラで処理・処分・資源化~
錯体化学・溶液化学に基づく核燃料サイクル先進基盤研究
鷹尾研究室では、ウランをはじめとしたアクチノイド元素および様々な関連元素の錯体化学および溶液化学に基づき、いわゆる【核のゴミ】である使用済み核燃料や高レベル放射性廃棄物などの適切な処理・処分ならびに資源化を目指しています。
昨今、原子力にまつわるニュース・話題として例えば廃炉・廃止措置・放射性廃棄物処理および処分など、どちらかというと一般にはネガティブな印象を生むワードを含むものが多い点が否めません。原子力エネルギーの恩恵を享受してきた現代社会全体の責任としてこれらの重要課題に取り組んでいかなければならないのは確かですが、最初から後始末に特化した研究開発だけではどうしても魅力に欠けてしまいます。これからの社会を担う将来ある若い世代の学生さん達には、単に応用だけでなく重要な基礎研究・学究の場である大学ならではの研究として、学術的価値が高くかつ将来の展望が大いに期待出来る夢のある研究テーマに楽しく(重要!)取り組んでもらいたいと考えています。
キーワード: 錯体化学, 溶液化学, アクチノイド化学, イオン液体, 金属錯体触媒, 光触媒, 核種分離, 核燃料サイクル, 使用済み核燃料再処理, 放射性廃棄物処理・処分
【卒研生・院生(M, D)募集中です。見学等随時受付。鷹尾までお気軽にお問い合わせください(連絡先は右上)。】
NEWS
|
2024/04/08 |
【メンバーページ更新】 Wangさん(特任助教)、佐藤(千)さん(D1)、上野君、小林君、下川君(以上M1)、佐藤(七)君(B4)を新メンバーとして迎えました。 【院試説明会・研究室見学会】 3/29(金)の研究室見学会に来てくれた学生さんたち、どうもありがとうございました。改めて個別に相談したいことがあれば、対面・zoomどちらでも対応できますのでお気軽に連絡ください。 今週末の4/13(土)にも物質理工学院研究室見学会を予定しています。今度はオンラインでの開催になります。鷹尾研に興味をお持ちの学生さんは、まずは見学会自体に事前登録をお願いします(前回3/29分に登録済みであれば4/13の情報も配信されていると思います)。アポなし飛び入りでも構いませんが、事前に連絡いただけるとよりスムーズに対応できると思います。 |
|
2024/04/02 |
【Paper Accepted】 津島先生との共著論文、"Towards tailoring hydrophobic interaction with uranyl(VI) oxygen for C-H activation"がChem. Commun.誌にアクセプトされました。 Tsushima, S.; Kretzschmar, J.; Doi, H.; Okuwaki, K.; Kaneko, M.; Mochizuki, Y.; Takao, K. "Towards tailoring hydrophobic interaction with uranyl(VI) oxygen for C-H activation", Chem. Commun. in press. DOI:10.1039/D4CC01030B 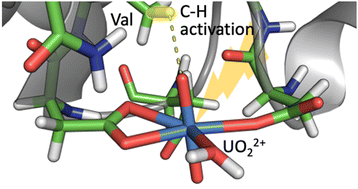 |
|
2024/03/29 |
【Photoおよび業績ページ更新】 荒木遼太君、伊藤広真君、折野匡君、NAM Taeyiさんが修士課程を小針拓己君が学士課程をそれぞれ修了しました。社会人になっても、もしくは進学しても明るく元気に頑張ってください!! 3/26-28に近畿大で開催された日本原子力学会2024年春の年会で小野、Nam, 田代、名畑、野島、鷹尾が計6件の口頭発表および田代、野島が2件のポスター発表を行ってきました。 【院試説明会・研究室見学会】 予定通り本日3/29(金)午後に物質理工学院研究室見学会を開催します。引き続き事前連絡受付中ですし、アポなしで直接立ち寄っていただいたのでも構いません。ただし、先に見学に来ている他の学生さんを実験棟などに案内中などで席を外しているかもしれません。不在の場合は大岡山北1号館205号室(鷹尾オフィス)前ホワイトボードを参照してください。 |
|
2024/03/08 |
【受賞!】 第19回(令和5年度)日本原子力学会 再処理・リサイクル部会各賞を以下の通りいただきました。3/26-28の日本原子力学会2024年春の年会再処理・リサイクル部会全体会議にて表彰されます。http://www.aesj.or.jp/~recycle/top.html ・業績賞 原子力エネルギーシステムの循環型経済確立に資する先端基礎化学研究およびアカデミアの役割に関する提言 鷹尾康一朗 ・優秀講演賞 ウラニル錯体化学に基づくテーラーメイド型新規海水ウラン吸着材開発 (3) H2saldian 型吸着材の開発 伊藤広真 アダマンタン骨格を架橋部位とするビス(2-ピロリドン)誘導体による難溶性アクチノイド(IV)-硝酸錯体の形成と沈殿法再処理への適用性 小野遼真 【院試関連】 各種進学説明会/研究室見学会の日程が決まりました。詳細については以下を参照してください。いずれも事前登録が必要なようです。見学・相談等対応しますので、鷹尾研に興味をお持ちの方はメール等で事前に連絡いただけると助かります。パンフレット等を見て当日飛び入りでも大丈夫です。 ・原子核工学コース(3/23(土), 5/11(土)):https://www.ne.titech.ac.jp/jp/examination/guidance.html ・物質理工学院(3/29(金, 対面), 4/13(土, zoom), 5/18(土, 対面)):https://www.titech.ac.jp/0/prospective-students/open-campus/briefing/school-materials-chemical-technology ※【院試・研究室生活などについて】も併せて参照ください。 |
|
2024/02/22 |
【Paper Accepted】 JAEA大内様, 小松様, 北辻様, 渡邉様との共著論文"Securing Reversibility of UVO2+/UVIO22+ Redox Equilibrium in [emim]Tf2N-Based Liquid Electrolytes towards Uranium Redox-Flow Battery"がEur. J. Inorg. Chem.誌に受理されました。レドックスフロー電池への劣化ウラン有効活用を目指した基礎研究の成果です。グラフィカルアブストラクトにうちのハムちゃん(チー太郎)がデビュー!! Takao. K.; Ouchi, K.; Komatsu, A.; Kitatsuji, Y.; Watanabe, M. "Securing Reversibility of UVO2+/UVIO22+ Redox Equilibrium in [emim]Tf2N-Based Liquid Electrolytes towards Uranium Redox-Flow Battery" Eur. J. Inorg. Chem. in press. DOI:10.1002/ejic.202300787.  |
|
2024/01/10 |
企業さんと共同で進めているプロジェクトに携わる特任助教を募集しています。詳細については以下のURLおよび公募案内PDFをご覧ください。 |
|
2023/12/14 |
【院試説明会・研究室見学会】 〇原子核工学コース(応化系・融合理工学系)大学院進学説明会 ・日時:2024/1/20(土) 13:00-15:30頃 (3/23, 5/11にも開催予定) ・場所:対面(大岡山北1号館1階会議室)・オンライン(zoom)併用 ※要事前登録(コチラから)、現地参加の場合は説明会後に研究室見学可(見学希望の旨メール連絡をいただけると助かります) ・URL:http://www.ne.titech.ac.jp/jp/examination/guidance.html |
|
2023/11/22 |
【Photoページ更新】 11/6-17の期間、ドイツ・ドレスデンのHelmholtz-Zentrum Dresden-Rossendorf (HZDR)にてPuを用いた実験を行ってきました。我々が開発を進める核燃料物質選択的沈殿法(NUMAP法)による使用済み核燃料次世代再処理技術開発に欠かせない貴重なデータを多数取得することに成功しました。出来る限り早期の論文化を目指します。 |
|
2023/08/31 |
【Paper Accepted】 竹山君(2023年3月博士修了, 現:山口東京理科大助教)、津島先生、HZDRメンバー(Dr. R. Gericke, Dr. P. Kaden, Dr. J. Mӓrz)との共著論文"Fate of Oxidation States at Actinide Centers in Redox-Active Ligand Systems Governed by Energy Levels of 5f Orbitals"がChem. Eur. J.誌に受理されました。 Takeyama, T.; Tsushima, S.; Gericke, R.; Kaden, P.; Mӓrz, J.; Takao, K. "Fate of Oxidation States at Actinide Centers in Redox-Active Ligand Systems Governed by Energy Levels of 5f Orbitals", Chem. Eur. J. 2023, e202302702. DOI:10.1002/chem.202302702  MITからの海外交流学生Alonso君が予定の滞在日程を終えてアメリカに帰国しました。9月からすぐに新学期が始まるということで、休む暇もないけど頑張ってください。 |
|
2023/08/17 |
【Paper Accepted】 山本さん(2022年度鷹尾研研究員, 現:東工大応化系山中研助教)らとの共著論文" "Solid-State Schikorr Reaction from Ferrous Chloride to Magnetite with Hydrogen Evolution as the Kinetic Bottleneck"がInorg. Chem.誌に受理されました。 Yamamoto, M.; Takamura, Y.; Kokubo, Y.; Urushihara, M.; Horiuchi, N.; Dai, W.; Hayasaka, Y.; Kita, E.; Takao, K. "Solid-State Schikorr Reaction from Ferrous Chloride to Magnetite with Hydrogen Evolution as the Kinetic Bottleneck" Inorg. Chem. 2023, 62, 14580-14589. DOI:10.1021/acs.inorgchem.3c01676.  |
|
2023/07/26 |
【HOT Article Collection選出!】【Front Cover掲載!】 Dalton Trans.誌から出版された私のPerspective論文"How does chemistry contribute to circular economy in nuclear energy systems to make them more sustainable and ecological?" が、HOT Article Collectionに選ばれました。無機化学分野において興味深くかつ顕著であると評されたTop 10%の論文が四半期ごとに選出されるそうです。6週間アクセスフリーとのことなので、ぜひご一読ください。 また、この論文はFront Coverにも選出されており、同誌Vol. 52, Issue 29の表紙を飾りました。  |
| 2023/06/05 |
マサチューセッツ工科大 学部3年生のPedro Alonso Hernzndez君を海外交流学生として迎えました。 今年度院試のインターネット出願サイトでの情報登録が始まりましたね(出願書類紙媒体一式の提出は6/8~6/14必着、詳細については募集要項記載内容に従ってください)。 趣味のページなので都度アナウンスしてはいませんが、釣行記録やハムちゃんの写真もちょいちょいアップデートしています。 |
|
2023/05/29 |
5/27(土)の物質理工学院研究室見学会含め、これまでに見学・相談に来てくれた学生さんたち、どうもありがとうございました。6/3(土)午後にも原子核工学コースの進学説明会が予定されていますが、こちらについては申し訳ないことに都合により欠席する予定です。出願期間(6/8~14,
募集要項を参照のこと)までまだもう少しだけ日数がありますので、見学や相談をご希望の場合はメール等で個別に連絡してください。 また、今年度は学部3年生以下の学生さんの訪問も結構多かったように思います。学内外および学年問わず見学・相談を受け付けていますので、ぜひどうぞ! 【院試・研究室生活などについて】も併せてご覧ください。 |
|
2023/05/18 |
【院試説明会・研究室見学会】 物質理工学院応用化学系の研究室見学会が以下の通り開催されます。 ・日程:2023/5/27(土, 対面) 13:00-17:00 ※見学希望の方は、前日(5/26(金))までにメールにて連絡ください ・場所:大岡山北1号館205(鷹尾居室) ※初回のみこちらから要事前登録とのこと。例年通りなら研究室一覧HPやzoom情報など見学会の案内などが配信されるようです。 ・URL 全体:https://www.titech.ac.jp/0/prospective-students/open-campus/briefing/school-materials-chemical-technology 5/27(土):https://www.titech.ac.jp/0/prospective-students/news/2023/066314 【院試・研究室生活などについて】も併せてご覧ください。 |
|
2023/04/26 |
【Paper Accepted】 Dalton Trans.誌からの依頼で執筆したPerspective論文"How does chemistry contribute to circular economy in nuclear energy systems to make them more sustainable and ecological?"がアクセプトされました。初めて書いた単著論文。本来であれば循環型経済(Circular Economy)の概念に合致するはずである核燃料サイクルにおける現状の課題、鷹尾研で進めているいくつかの主な研究テーマも含め化学特に錯体化学研究がその中でどのような位置づけを占めるのか、また基礎化学研究に携わる我々アカデミアの果たすべき役割として私の信ずるところ、などをまとめたPerspective論文(日本語だと"見通し, 展望, 大局観"くらい?和訳しにくい…)です。 鷹尾研の研究内容を網羅しきれてはいないのですが、どういう背景・コンセプト・スタンスで研究を行っているのかを端的にまとめてあります。読んでみたい方にはPDFを送りますので気軽に連絡ください。もちろん英語ですが、査読時のレフリーコメントでは読みやすいと結構好評でした。 Takao, K. "How does chemistry contribute to circular economy in nuclear energy systems to make them more sustainable and ecological?" Dalton Trans. 2023, 52, 9866-9881. doi:10.1039/D3DT01019H [Perspective] 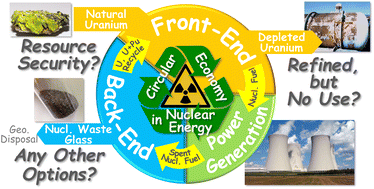 【院試説明会・研究室見学会】 原子核工学コース進学説明会が以下の通り開催されます。 ・日程:2023/5/14(日, オンライン) 13:00-15:30頃 ・場所:オンライン(zoom) ※要事前登録(以下のページに登録リンクがあります) ・URL:http://www.ne.titech.ac.jp/jp/examination/guidance.html 【5/14進学説明会に来てくれた学生さんたち、どうもありがとうございました。改めて個別に相談したいことがあればお気軽にご連絡ください】 |
|
2023/04/14 |
明日4/15(土)午後に物質理工学院研究室見学会が開催されます。といっても今回はオンラインです。事前登録後に案内のあったHPに記載の通り、当日でも構わないので可能であれば事前に私宛にメールをいただけると助かります。連絡は訪問直前(もしくはアポなし)でも構いませんが、一時的に席を外しているかもしれないのでその場合は戻るまで少しお待ちください。 |
|
2023/04/03 |
小久保さん(博士研究員→5月から特任助教)、田代さん、名畑君、野島君(以上M1)、小針君(B4)を新メンバーとして迎えました。 →【メンバー】ページ更新 3/30(木)の研究室見学会に来てくれた学生さんたち、どうもありがとうございました。改めて個別に相談したいことがあれば、対面・zoomどちらでも対応できますのでお気軽に連絡ください。 |
|
2023/03/29 |
竹山知志君が博士課程、内田昇吾君と奥村優太君が修士課程をそれぞれ修了しました。社会に出ても明るく元気に頑張ってください!! 【院試説明会・研究室見学会】 予定通り明日3/30(木)午後に物質理工学院研究室見学会を開催します。引き続き事前連絡受付中ですし、当日アポなしで来ていただいても構いません。ただし、先に見学に来ている他の学生さんを実験棟などに案内しているかもしれないので、不在の場合は大岡山北1号館205号室(私の居室)前のホワイトボードに記載してある番号までお電話ください。 |
|
2023/03/16 |
【院試説明会・研究室見学会】 物質理工学院応用化学系の研究室見学会が以下の通り開催されます。 ・日程:2023/3/30(木, 対面), 4/15(土, オンライン) 両日とも13:00-17:00 ※5/27(土)も予定(開催形式?) ・場所:大岡山北1号館205(鷹尾居室) zoomでも対応可 ※初回のみこちらから要事前登録とのこと。例年通りなら研究室一覧HPやzoom情報など見学会の案内などが配信されるようです。 ・URL 全体:https://www.titech.ac.jp/0/prospective-students/open-campus/briefing/school-materials-chemical-technology 3/30(木):https://www.titech.ac.jp/0/prospective-students/news/2023/066168 4/15(土):https://www.titech.ac.jp/0/prospective-students/news/2023/066170 両日とも出席予定ですので、見学・相談などお気軽にどうぞ。事前に連絡いただけるとスムーズに対応できますが、アポなしで気軽に来ていただいたので構いません。 【院試・研究室生活などについて】も併せてご覧ください。 |
|
2023/03/08 |
【Paper Accepted】 竹山君(D3)、津島先生との共著論文"Utility of redox-active ligands for reversible multi-electron transfer in uranyl(VI) complexes"がInorg. Chem. Frontiers誌に受理されました。この論文は同誌Vol. 10, Issue 14のフロントカバー(表紙)を飾っています。 Inorg. Chem. Frontiers, 2023, 10, 4028-4044. doi: 10.1039/D3QI00189J  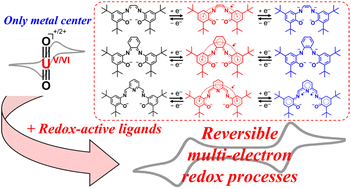 |
|
2023/02/27 |
【Paper Accepted】 竹山君(D3)、小野君(D1)、Dr. März (HZDR, ドイツ)、津島先生との共著論文、"Neptunyl(VI) Nitrate Coordination Polymer with Bis(2-pyrrolidone) Linkers to Highlight Crystallographic Analogy and Solubility Difference in Actinyl(VI) Nitrates"がInorganics誌に受理されました。 Inorganics, 2023, 11, 104. doi: 10.3390/inorganics11030104 [Open Access]  |
| 2022年12月20日 |
【院試説明会・研究室見学会】 原子核工学コース(応化系・融合理工学系)の大学院進学説明会が以下の通り開催されます。 ・日時:2023年1月21日(土) 13:00~15:30 その後個別相談可 ・場所:オンライン(zoom) *要事前登録 ・URL:http://www.ne.titech.ac.jp/jp/examination/guidance.html 鷹尾も出席予定ですので、見学・相談などお気軽にどうぞ(アポなしでもOKです)。 |
|
2022年12月15日 |
【Paper Accepted】 小野君(D1)、風間君(鷹尾研OB, 現JAEA)、Dr. März (HZDR, ドイツ)、津島先生との共著論文、"Crystal Structures of Ce(IV) Nitrates with Bis(2-pyrrolidone) Linker Molecules Desposited from Aqueous Solutions with Different HNO3 Concentrations"がInorg. Chem.誌に受理されました。 Inorg. Chem. 2023, 62, 454-463. doi: 10.1021/acs.inorgchem.2c03554  |
|
2022年10月28日 |
10/9-23の期間、ドイツとフランスでそれぞれ開催されたHZDR Innvation & Exchange
WeekとATAS-AnXAS Workshopに出張してきました。久しぶりの対面会議で非常に有意義でした。 【Photo】ページを更新  |
|
2022年10月3日 |
山本さんとの共著論文"Non-aqueous bonding of leuprorelin to Ochratoxin
A for peptide-based solid-phase extraction"がChem.
Commn.誌に受理されました。 Chem. Commn. 2022, 58, 12106-12109. doi:10.1039/D2CC04430G |
|
2022年9月22日 |
学位記授与式が執り行われました。Zheng Zhiwei君(Weber)と曹月明さん(Cao
Yueming)がそれぞれ博士および修士課程を修了しました。社会に出ても明るく元気に頑張ってください!(次の勤務先のvisaの関係で、Weberはポスドクとしてもうしばらく研究室にいるけど) 【Photoページ】更新 |
|
2022年8月26日 |
鷹尾が共同執筆の「イオン液体の実用展開へ向けた最新動向」がシーエムシー出版から出版されました。第9章3節「イオン液体を用いた核燃料サイクル・放射性廃棄物処理」の執筆を担当。 https://www.cmcbooks.co.jp/products/detail.php?product_id=8637 |
|
2022年8月22日 |
【Paper Accepted】 竹山君(D3)との共著論文"Effects of coordinating heteroatoms on molecular structure, thermodynamic stability and redox behavior of uranyl(VI) complexes with pentadentate Schiff-base ligands"がRSC Adv.誌に受理されました。RSC Adv. 2022, 12, 24260-24268. doi:10.1039/D3RA04639C [Open Access]  |
|
2022年5月17日 |
4/25~5/5の期間、ドイツのHelmholtz Zentrum
Dresden-Rossendorfにて念願だったPu(IV)を用いた実験を行ってきました。コロナ禍による出入国時の水際対策もすべてクリアし、無事完遂しました。 【Photo】ページ更新 以前発表したDr. Maerz (HZDR). 津島先生らとの共著論文 #76 RSC Adv. 2020, 10, 6082-6087がEditor's Collection "Shining a Light on the f-Block"に選出  |
| 2022年4月6日 |
【Paper Accepted】 竹山君(D3), 岩月先生(甲南大), 津島先生との共著論文"Synthesis and characterization of uranyl(VI) complex with 2,6-pyridine-bis(methylaminophenolato) and its ligand-centred aerobic oxidation mechanism to diimino derivative"がDalton Trans.誌に受理されました。Dalton Trans. 2022, 51, 6576-6586. doi:10.1039/D2DT00325B 【Outside Back Coverに採用】  |
|
2022年4月1日 |
小野遼真君、水町匠君、Randrianasolo
Falitianaさん、佐藤みなみさんが修士課程を修了しました。小野君は博士課程で、他3名は社会に出ても明るく元気に頑張ってください。 伊藤広真君、折野匡君、南泰伊さん(以上新M1)、山本雅納さん(博士研究員)をを新メンバーとして迎えました。(2022年度研究室体制:スタッフ3名+学生10名) |
|
2022年3月29日 |
【Paper Accepted】 水町君(2021年度修士修了)、佐藤さん(2021年度修士修了)、金子様(JAEA)、D2竹山君、津島先生との共著論文"Fully Chelating N3O2-Pentadentate Planar Ligands Designed for Strongest and Selective Capture of Uranium from Seawater"がInorg. Chem.誌に受理されました。Inorg. Chem. 2022, 61, 6175-6181. doi:10.1021/acs.inorgchem.2c00306  |
|
2022年3月18日 |
鷹尾と池田先生の連名で日本原子力学会再処理・リサイクル部会業績賞をいただきました。 日本原子力学会再処理・リサイクル部会業績賞, "簡易・高汎用次世代再処理技術としての核燃料物質選択的沈殿法の開発", 東京工業大学 鷹尾・池田研究室. 【再処理・リサイクル部会HP】  |
|
2022年3月16日 |
3/30(水)の物質理工学院研究室見学会は対面形式で開催するそうです。鷹尾研の見学を希望される学生さんは以下のページ内にあるエントリーフォームのリンクから事前登録をお願いします(1度でOKです)。 https://www.titech.ac.jp/0/prospective-students/news/2022/063239 (3/30分, 対面形式) https://www.titech.ac.jp/0/prospective-students/news/2022/063697 (4/16分, 詳細については上記説明会URLもしくは【院試・研究室生活】ページを参照ください。 |
|
2022年3月14日 |
【Paper Accepted】 成蹊大坪村先生との共著論文"Design and Demonstration of Absorption/Luminescence-Compatible Spectroelectrochemical Cell with Optically Transparent Thin-Layer Electrode"がJ. Electroanal. Chem.誌に受理されました。J. Electroanal. Chem. 2022, 911, 116217. doi:0.1016/j.jelechem.2022.116217  |
|
2022年1月19日 |
【Paper Accepted】 津島先生との共著論文"Hydrophobic Core Formation and Secondary Structure Elements in Uranyl(VI)-Binding Peptides"がPhys. Chem. Chem. Phys.誌に受理されました。Phys. Chem. Chem. Phys. 2022, 24, 4455-4461, [Open Access] doi:10.1039/D1CP05401E  |
|
2022年1月11日 |
新年あけましておめでとうございます。今年もよろしくお願いいたします。 原子核工学コース(応化系・融合理工学系)の大学院進学説明会が以下の通り開催されます。 ・日時:2022年1月22日(土) 13:00~15:00 その後研究室見学 ・場所:オンライン(zoom) *要事前登録 ・URL:http://www.ne.titech.ac.jp/jp/examination/guidance.html 鷹尾も出席予定ですので、見学・相談などお気軽にどうぞ(アポなしでもOKです)。 → 【研究室生活など】進学説明会情報を更新 |





