Visualization of the recruitment kinetics of DNA repair factor PNKP to DNA damages at seconds-scale
2020.11.2
Highlights
- Demonstrating the recruitment kinetics of PNKP and related proteins to laser-induced DNA damages at seconds-scale
- Arg35/Arg48 of PNKP are structurally important for protein-protein interactions in a phosphorylation-dependent manner
- Mutations of Arg35/Arg48 impaired its accumulation to DNA damage sites, the ability of SSB and DSB repair, and the maintenance of genome stability
Summary
The study revealed that the recruitment kinetics of DNA repair factor “PNKP” at the sites of DNA damages, and which is strictly regulated in seconds by protein-protein interactions in a phosphorylation-dependent manner. Moreover, these mechanisms are required for the cell survival and the maintenance of genome stability.
Mr. Kaima Tsukada (first author), Dr. Mikio Shimada (corresponding author), Mr. Rikiya Imamura, Mr. Kotaro Saikawa, Dr. Yoshihisa Matsumoto at Matsumoto laboratory, Laboratory for Advanced Nuclear Energy, Institute of Innovative Research, Tokyo Institute of Technology, and Dr. Masamichi Ishiai at Research Institute, National Cancer Center, were conducted this research project. This study was published in the scientific journal " Mutation Research -Fundamental and Molecular Mechanisms of Mutagenesis-" from ELSEVIER.
Background
Our genomic information is conserved by deoxyribonucleic acids (DNA), and genome DNA (gDNA) is always damaged due to endogenous factors (i.e. DNA replication errors and active oxygen generated by intracellular metabolism) and exogenous factors (i.e. ultraviolet light and ionizing radiation). These damages are immediately repaired by the DNA repair machinery to prevent genetic mutations.
Polynucleotide Kinase Phosphatase (PNKP) is one of DNA repair factors which has dual enzymatic activities as kinase and phosphatase for DNA ends, consists of 3 domains (FHA, phosphatase and kinase) and a linker region.
In this study, we established the system “laser micro-irradiation” which can induce regional DNA damage in the part of cell nucleus and visualize the DNA repair dynamics in a living cells at seconds-scale. Using this system, we analyzed the importance of the FHA domain of PNKP in the its recruitment at the laser-induced DNA damages, DNA repair abilities and cell survivals.
Results
We firstly aligned and analyzed amino acid sequences of PNKP among a variety of species, and the in silico identified central amino acids of the FHA domain Arg35 and Arg48, which are structurally important (Figure1).
To analyze the importance of Arg35/Arg48 in the DNA repair dynamics, we constructed GFP-PNKP fusion protein and substituted protein (R35A/R48A) and performed the laser micro-irradiation assay.
The laser micro-irradiation assay revealed that PNKP is accumulated at laser-induced DNA damages in seconds and substitution of R35/R48 resulted in the defected accumulation (Figure2).
Moreover, we analyzed the DNA repair ability in PNKP R35A/R48A mutant after ionizing radiation (IR) exposure by immuno-fluorescence staining using an ADP-ribose binding regent for the detection of DNA single-strand breaks and γ-H2AX antibody for the detection of DNA double-strand breaks. PNKP R35A/R48A mutant showed significantly impaired DNA repair abilities in both of DNA single-strand break repair and DNA double-strand break repair compared to cells expressing PNKP WT. Moreover, R35A/R48A mutant exhibited higher sensitivity to IR exposure and increased number of micronuclei formation which represents genome instability.
Taken together, we bioinformatically analyzed the FHA domain of PNKP and therein identified the central amino acids R35 and R48, which are required for the phosphorylation-dependent protein-protein interactions, the accumulation of PNKP at DNA damage sites, efficient DNA repair, and preventing genome instability.
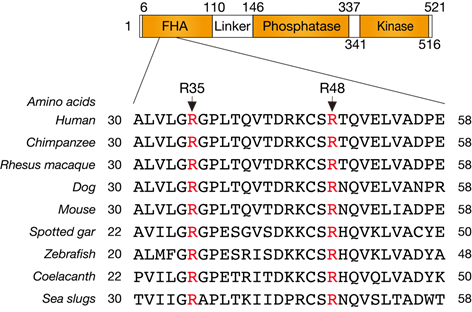
Figure1 Alignment of PNKP amino acid sequences among a variety of species
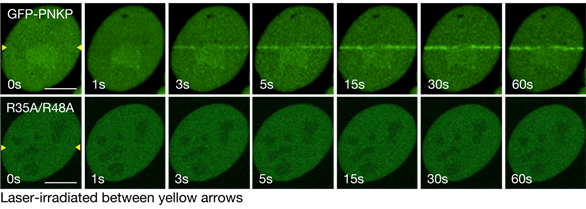
Figure2 Regulatory mechanisms of PNKP for its accumulation at laser-induced DNA damages
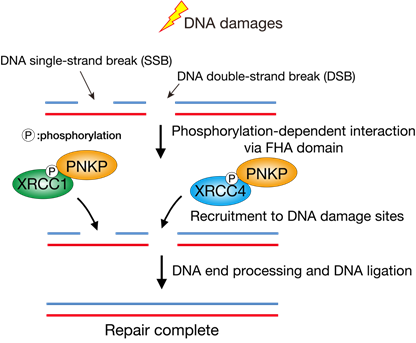
Figure3 The PNKP accumulation at DNA damage sites regulated by protein-protein interactions
Journal information
- Journal :
- Mutation Research -Fundamental and Molecular Mechanisms of Mutagenesis-
- Title of original :
- The FHA domain of PNKP is essential for its recruitment to DNA damage sites and maintenance of genome stability
- Author :
- Kaima Tsukada (first author)
Mikio Shimada (corresponding author)
Rikiya Imamura
Kotaro Saikawa
Masamichi Ishiai
Yoshihisa Matsumoto
